An improved analytical model of protein dynamics at the sub-nanosecond timescale
Abstract
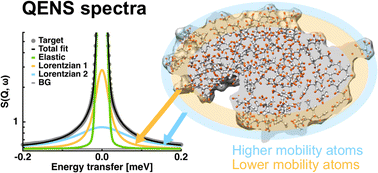
Thermal fluctuations of proteins at the ps–ns timescales are important for their functions and have extensively been studied using quasi-elastic neutron scattering (QENS). In general, the QENS spectra of proteins are analyzed in terms of two populations of atoms, i.e., the immobile fraction of atoms, the motions of which are too slow to be resolved within the instrumental energy resolution employed, and the mobile fraction of atoms, from which the average amplitude and frequency of protein atomic motions are characterized. On the other hand, it has been shown using molecular dynamics simulations that atomic motions are gradually enhanced as going from the protein core to the protein surface. Therefore, it is essential to further deconvolute the mobile fraction of atoms to study in detail the dynamical behavior of proteins. Here, an improved analytical model using QENS to deconvolute the mobile fraction of atoms into two populations of atoms, i.e., atoms with high mobility (HM atoms) and those with low mobility (LM atoms), is proposed. It was found that both the HM atoms and the LM atoms showed gradually enhanced dynamics with an increase in temperature even though any temperature-dependent terms are not included in the model. The presented model yields physically reasonable values for dynamical parameters and hence its future application will be useful to understand the molecular mechanism of various protein functions where atoms with higher mobility on or close to the protein surface play a crucial role.


 Please wait while we load your content...
Please wait while we load your content...