Silver cation tagged on 5,7,12,14-tetraphenyl-6,13-diazapentacene and its dihydro-form†
Abstract
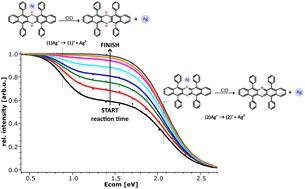
The attachment of silver(I) cations to 5,7,12,14-tetraphenyl-6,13-diazapentacene and its reduced dihydro-form has been studied by electrospray ionization mass spectrometry (ESI-MS). The structure elucidation of the Ag+ complexes has been accomplished in gas-phase collision experiments in conjunction with density functional theory (DFT) calculations. The oxidized form provides a favourable cavity for the Ag+ ion, leading to the [1 : 1] complex with the highest resilience towards dissociation and severely hindering the attainment of a second molecular ligand. When the nitrogen is hydrogenated in the reduced dihydro-form, the cavity is partly blocked. This leads to a less strongly bound [1 : 1] complex ion but facilitates the attachment of a second molecular ligand to the Ag+. The resulting complex is the most stable among the [2 : 1] complexes. DFT calculations provide valuable insight into the geometries of the complex ions. Adding silver(I) to the reduced dihydro-form for cationization also induces its oxidation in solution. The oxidative dehydrogenation reaction, for which a mechanism is proposed, proceeds by first order kinetics and is markedly accelerated by day light.


 Please wait while we load your content...
Please wait while we load your content...