Multifunctional fluorescent diarylethene: time-resolved study of photochemistry†
Abstract
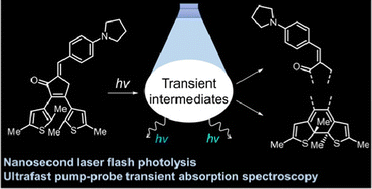
A study of luminescence and photochromic properties of (E)-2,3-bis(2,5-dimethylthiophen-3-yl)-5-(4-(pyrrolidin-1-yl)benzylidene)cyclopent-2-en-1-one, which is a diarylethene with a push–pull system between carbonyl and dimethylamino groups, was performed using time-resolved methods. The intramolecular charge transfer (ICT) process as well as 6π-electrocyclization and E-/Z-isomerization contribute to the complex light-induced properties of this molecule. Formation of unexpected short-lived intermediates was detected in the time range from 100 fs to 100 μs. A model based on two processes (additional photocyclization and interconversion between conformers) was proposed to rationalize this result. The key intermediates existing in the picosecond time domain are so-called precursors, which are proposed for both parallel (p) and anti-parallel (ap) isomers of the open form. In general, fast light-induced processes for the fluorescent diarylcyclopentenones are much more complicated than for the parent cyclopentenone-based DAE.


 Please wait while we load your content...
Please wait while we load your content...