Protein charge transfer spectra in a monomeric protein with no lysine†
Abstract
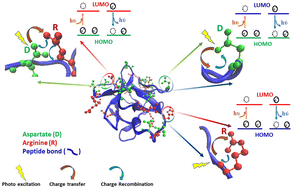
UV-Visible absorption and luminescence originating from non-aromatic groups in proteins is being intensely investigated today. Earlier work has shown that non-aromatic charge clusters in a folded monomeric protein can collectively act like a chromophore. Incident light in the near UV-Visible wavelength causes photoinduced electron transfer from the Highest Occupied Molecular Orbital (HOMO) of the electron-rich donor (like a carboxylate anion) to the Lowest Unoccupied Molecular Orbital (LUMO) of the electron-deficient acceptor (like a protonated amine or the polypeptide backbone) in the protein giving rise to absorption spectra in the 250–800 nm range referred to as Protein Charge Transfer Spectra (ProCharTS). The transferred electron can relax back from the LUMO to fill up the hole in the HOMO by a charge recombination process, emitting weak ProCharTS luminescence. Previous studies in monomeric proteins displaying ProCharTS absorption/luminescence always involved lysine-containing proteins. The lysine (Lys) sidechain plays a dominant role in ProCharTS; however, experimental evidence for ProCharTS among proteins/peptides devoid of Lys is lacking. Recently, the absorption features of charged amino acids have been examined using time-dependent density functional theory calculations. In this study, we show that amino acids: arginine (Arg), histidine (His) and aspartate (Asp); homo-polypeptides: poly-arginine and poly-aspartate; and a protein: Symfoil PV2 that is rich in Asp, His and Arg, but lacks Lys, profusely display ProCharTS. The folded Symfoil PV2 protein displayed maximum ProCharTS absorptivity in the near UV-Vis region, in comparison to the homo-polypeptides and amino acids. Furthermore, features like overlapping ProCharTS absorption spectra, decreasing ProCharTS luminescence intensity with longer excitation wavelength, large Stokes shift, multiple excitation bands and multiple luminescence lifetime components appeared to be conserved across peptides, proteins and amino acids studied. Our results underscore the utility of ProCharTS as an intrinsic spectral probe to monitor the structure of any protein that is rich in charged amino acids.


 Please wait while we load your content...
Please wait while we load your content...