Anion and ether group influence in protic guanidinium ionic liquids†
Abstract
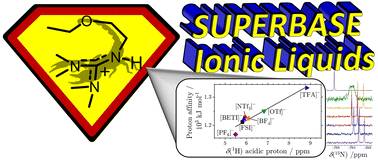
Ionic liquids are attractive liquid materials for many advanced applications. For targeted design, in-depth knowledge about their structure–property-relations is urgently needed. We prepared a set of novel protic ionic liquids (PILs) with a guanidinium cation with either an ether or alkyl side chain and different anions. While being a promising cation class, the available data is insufficient to guide design. We measured thermal and transport properties, nuclear magnetic resonance (NMR) spectra as well as liquid and crystalline structures supported by ab initio computations and were able to obtain a detailed insight into the influence of the anion and the ether substitution on the physical and spectroscopic properties. For the PILs, hydrogen bonding is the main interaction between cation and anion and the H-bond strength is inversely related to the proton affinity of the constituting acid and correlated to the increase of 1H and 15N chemical shifts. Using anions from acids with lower proton affinity leads to proton localization on the cation as evident from NMR spectra and self-diffusion coefficients. In contrast, proton exchange was evident in ionic liquids with triflate and trifluoroacetate anions. Using imide-type anions and ether side groups decreases glass transitions as well as fragility, and accelerated dynamics significantly. In case of the ether guanidinium ionic liquids, the conformation of the side chain adopts a curled structure as the result of dispersion interactions, while the alkyl chains prefer a linear arrangement.


 Please wait while we load your content...
Please wait while we load your content...