The magnetic properties of MAl4(OH)12SO4·3H2O with M = Co2+, Ni2+, and Cu2+ determined by a combined experimental and computational approach†
Abstract
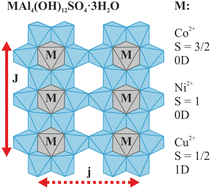
The magnetic properties of the nickelalumite-type layered double hydroxides (LDH), MAl4(OH)12(SO4)·3H2O (MAl4-LDH) with M = Co2+ (S = 3/2), Ni2+ (S = 1), or Cu2+ (S = 1/2) were determined by a combined experimental and computational approach. They represent three new inorganic, low-dimensional magnetic systems with a defect-free, structurally ordered magnetic lattice. They exhibit no sign of magnetic ordering down to 2 K in contrast to conventional hydrotalcite LDH. Detailed insight into the complex interplay between the choice of magnetic ion (M2+) and magnetic properties was obtained by a combination of magnetic susceptibility, heat capacity, neutron scattering, solid-state NMR spectroscopy, and first-principles calculations. The NiAl4- and especially CoAl4-LDH have pronounced zero-field splitting (ZFS, easy-axis and easy-plane, respectively) and weak ferromagnetic nearest-neighbour interactions. Thus, they are rare examples of predominantly zero-dimensional spin systems in dense, inorganic matrices. In contrast, CuAl4-LDH (S = 1/2) consists of weakly ferromagnetic S = 1/2 spin chains. For all three MAl4-LDH, good agreement is found between the experimental magnetic parameters (J, D, g) and first-principles quantum chemical calculations, which also predict that the interchain couplings are extremely weak (< 0.1 cm−1). Thus, our approach will be valuable for evaluation and prediction of magnetic properties in other inorganic materials.


 Please wait while we load your content...
Please wait while we load your content...