Adsorption of CO32−/HCO3− on a quartz surface: cluster formation, pH effects, and mechanistic aspects†
Abstract
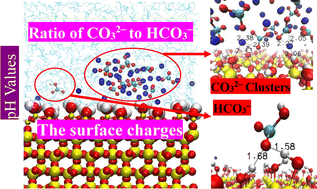
Soluble inorganic carbon is an important component of a soil carbon pool, and its fate in soils, sediments, and underground water environments has great effects on many physiochemical and geological processes. However, the dynamical processes, behaviors and mechanism of their adsorption by soil active components, such as quartz, are still unclear. The aim of this work is to systematically address the anchoring mechanism of CO32− and HCO3− on a quartz surface at different pH values. Three pH values (pH 7.5, pH 9.5 and pH 11) and three carbonate salt concentrations (0.07, 0.14 and 0.28 M) are considered, and molecular dynamics methods are used. The results indicate that the pH value regulates the adsorption behavior of CO32− and HCO3− on the quartz surface by affecting the CO32−/HCO3− ratio and the surface charge of quartz. In general, both HCO3− and CO32− ions were able to adsorb on the quartz surface and the adsorption capacity of CO32− is higher than that of HCO3−. HCO3− ions tended to uniformly distribute in an aqueous solution and contact the quartz surface in the form of single molecules instead of clusters. In contrast, CO32− ions were mainly adsorbed as clusters which became larger as the concentration increased. Na+ ions were essential for the adsorption of HCO3− and CO32−, because some of the Na+ and CO32− ions spontaneously associated together to form clusters, promoting the clusters to be adsorbed on the quartz surface through cationic bridges. The local structures and dynamics trajectory of CO32− and HCO3− showed that the anchoring mechanism of carbonate solvates on quartz involved H-bonds and cationic bridges, which changed in relation to the concentration and pH values. However, the HCO3− ions mainly adsorbed on the quartz surface via H-bonds while the CO32− ions tended to be adsorbed through cationic bridges. These results may help in understanding the geochemical behavior of soil inorganic carbon and further the processes of the Earth's carbon chemical cycle.


 Please wait while we load your content...
Please wait while we load your content...