Theoretical understanding of stability of mechanically interlocked carbon nanotubes and their precursors†
Abstract
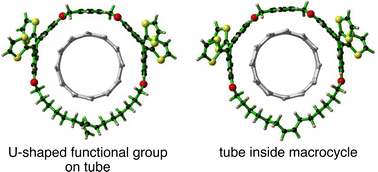
Dispersion-corrected DFT calculations were performed on (a,a) nanotubes (a = 5–10) attached by a U-shaped functional group consisting of p-xylene-linked double 9,10-di(1,3-dithiol-2-ylidene)-9,10-dihydro anthracene terminated by CnH2n chains (n = 6, 8, and 9), and their ring-closing macrocycles containing tubes. The reactant precursors and macrocycles are denoted by UP-n-(a,a) and (a,a)@Cycle-n, respectively. We found that UP-n-(a,a) are energetically preferable relative to the dissociation limit toward a U-shaped functional group (UP-n) and a tube (initial state) due to the attractive CH–π and π–π interactions. The attractive interactions are enhanced by increasing the tube diameters and CnH2n chain lengths because UP-n structures can be easily adjusted to interact with the tubes. The stability of (a,a)@Cycle-n and related (a,b)@Cycle-n is sensitive to tube diameters due to the restriction of ring structures. When diameter differences between a Cycle-n and a tube (D–d) are larger than 5 Å, (a,a)@Cycle-n plus C2H4 are energetically preferable relative to the initial state. However, the (a,a)@Cycle-n plus C2H4 byproduct is always energetically unstable relative to UP-n-(a,a). The DFT calculations found that the energy differences were low at D–d values ranging from 7 to 8 Å, explaining the tube-diameter-selective formation of the mechanically-interlocked tubes, observed experimentally.


 Please wait while we load your content...
Please wait while we load your content...