The effect of substituents and molecular aggregation on the room temperature phosphorescence of a twisted π-system†
Abstract
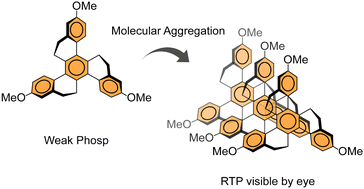
Considering the relevance of room temperature phosphorescent (RTP) materials, we discuss the influence of donor and acceptor groups substituted on to a twisted three-fold symmetric hydrocarbon homotruxene, which presents a persistent RTP, even in the absence of donor or acceptor moieties, under ambient conditions as a result of the twisted π-system. Compared to a fluorine acceptor, a donor methoxy group increases the phosphorescence decay rate in solution, while in the solid-state, molecular aggregation and packing yield a very persistent phosphorescence visible by the eye. The RTP of the intrinsically apolar homotruxene is found to be modulated by polar substituents, whose main impact on the solid-state emission is due to altered packing in the crystal.


 Please wait while we load your content...
Please wait while we load your content...