Solvation structures of calcium and magnesium ions in water with the presence of hydroxide: a study by deep potential molecular dynamics†
Abstract
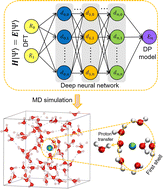
The solvation structures of calcium (Ca2+) and magnesium (Mg2+) ions with the presence of hydroxide (OH−) ion in water are essential for understanding their roles in biological and chemical processes but have not been fully explored. Ab initio molecular dynamics (AIMD) is an important tool to address this issue, but two challenges exist. First, an accurate description of OH− from AIMD needs an appropriate exchange–correlation functional. Second, a long trajectory is needed to reach an equilibrium state for the Ca2+–OH− and Mg2+–OH− ion pairs in aqueous solutions. Herein, we adopt a deep potential molecular dynamics (DPMD) method to simulate 1 ns trajectories for the Ca2+–OH− and Mg2+–OH− ion pairs in water; the DPMD method provides efficient machine-learning-based models that have the accuracy of the SCAN exchange–correlation functional within the framework of density functional theory. The solvation structures of the cations and the OH− in terms of three different species have been systematically investigated. On the one hand, we find that OH− have more significant effects on the solvation structure of Ca2+ than that of Mg2+. We observe that the OH− substantially affects the orientation angles of water molecules surrounding the cation. Through the time correlation functions, we conclude that the water molecules in the first solvation shell of Ca2+ change their preferred orientation faster than those of Mg2+. On the other hand, with the presence of the cation in the first solvation shell of OH−, we find that the hydrogen bonds of OH− are severely altered, and the adjacent water molecules of OH− are squeezed. The two cations have substantially different effects on the solvation structure of OH−. Our work provides new insight into the solvation structures of Ca2+ and Mg2+ in water with the presence of OH−.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...