Semirigid discotic dimers: flexible but not flexible enough?†
Abstract
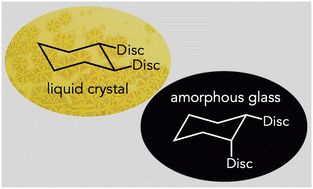
Two diastereomeric dibenzo[a,c]phenazine 1,2-cyclohexyl bridged diesters were prepared and their phase properties examined. While the trans dimer exhibited a broad columnar liquid crystal phase, the cis dimer was amorphous at all temperatures studied. This difference was attributed to the conformational dynamics of the two systems. NMR and DFT studies indicate that both dimers adopt folded and unfolded conformers in solution. While their folded geometries were similar, the trans dimer adopts an extended, largely planar structure, whereas the cis dimer is limited to non-planar unfolded structures and likely disrupts columnar ordering. Geometric constraints imposed by the cylcic linker were also important for columnar stability, with the trans dimer clearing 40 °C lower than the corresponding acyclic 2R,3R-butyl linked dimer, likely because the cyclohexyl group hinders π–π stacking in the unfolded conformation of the former.


 Please wait while we load your content...
Please wait while we load your content...