Forced topochemistry of a solid-state Diels–Alder reaction by encapsulation in epoxy glue†
Abstract
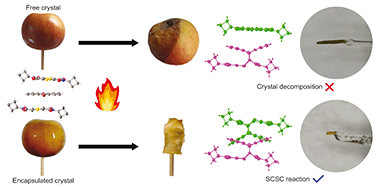
We report on the solid-state Diels–Alder thermal reaction in a 1 : 1 charge-transfer (CT) crystal composed of bis(N-cyclobutylimino)-1,4-dithiin (the electron acceptor) and 9-bromoanthracene (the electron donor) which crystallized in the triclinic space group, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The donor (D) and acceptor (A) molecules arrange in stacks where these molecules alternate, where a full 9-bromoanthracene donor molecule is surrounded by a symmetrically different acceptor molecule on the two molecular faces. The distance between the reacting atoms on the donor and the two acceptor molecules are slightly different but still within Schmidt's criteria, resulting in two reaction sites with different reaction environments, assigned as regions P and Q. Molecules in region P are more favourably aligned with the distances between reacting atoms being 3.51 Å and almost parallel as the molecules overlap each other. In region Q, the distances are 3.56 and 3.86 Å because the molecular overlap is more skewed, and the reacting atoms are rotated −15° from each other. Initially, the reaction occurs only in region P until ∼20% conversion is reached. Afterward, product Q is concurrently formed but at a slower rate. After ∼75% reaction, the crystal transforms from triclinic P
. The donor (D) and acceptor (A) molecules arrange in stacks where these molecules alternate, where a full 9-bromoanthracene donor molecule is surrounded by a symmetrically different acceptor molecule on the two molecular faces. The distance between the reacting atoms on the donor and the two acceptor molecules are slightly different but still within Schmidt's criteria, resulting in two reaction sites with different reaction environments, assigned as regions P and Q. Molecules in region P are more favourably aligned with the distances between reacting atoms being 3.51 Å and almost parallel as the molecules overlap each other. In region Q, the distances are 3.56 and 3.86 Å because the molecular overlap is more skewed, and the reacting atoms are rotated −15° from each other. Initially, the reaction occurs only in region P until ∼20% conversion is reached. Afterward, product Q is concurrently formed but at a slower rate. After ∼75% reaction, the crystal transforms from triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) into monoclinic C2/c, and conversion of ∼89% was found before the single crystal decomposes to become a powder. Reactions in free (unencapsulated) crystals above 10 °C were found to break apart during the initial reaction at around ∼20% when molecules in region P were reacting. Encapsulation of unreacted crystals with epoxy glue led to more reaction details being exposed and forced the reaction to occur topochemically until ∼89% conversion.
into monoclinic C2/c, and conversion of ∼89% was found before the single crystal decomposes to become a powder. Reactions in free (unencapsulated) crystals above 10 °C were found to break apart during the initial reaction at around ∼20% when molecules in region P were reacting. Encapsulation of unreacted crystals with epoxy glue led to more reaction details being exposed and forced the reaction to occur topochemically until ∼89% conversion.
- This article is part of the themed collection: Crystal Engineering in Africa


 Please wait while we load your content...
Please wait while we load your content...