Simulation of solid-state phase transition in dl-methionine†
Abstract
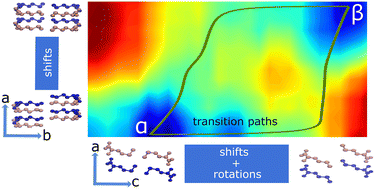
Solid-to-solid polymorphic transitions are a common phenomenon in organic crystals. The different interactions that play a role in these transitions are however far from understood. In this computational study, we aim to quantify the interactions that play a role in these transitions using the α ↔ β phase transition of DL-methionine as a model system. DL-Methionine has a layered structure and its phase transition occurs via shifting of the bilayers parallel to the b and c lattice vectors and the rotation of the side chains, which mostly affects the layers along the c lattice vector. We obtained two “order parameters” to describe the changes along b and c, respectively and that can be used to quantify the phase transition in terms of its thermodynamic parameters. The potential energy landscape is an interplay between van der Waals energy and configurational energy, where α is stabilized by configurational energy and destabilized by van der Waals energy as compared to β. The entropic contribution to the free energy difference between α and β is found to be in good agreement with experiments and completely dominated by configurational entropy.


 Please wait while we load your content...
Please wait while we load your content...