On the pairwise cocrystallization of racemic compounds†
Abstract
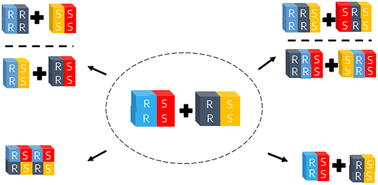
In the field of crystallization-based chiral resolution, preferential crystallization is particularly interesting. In recent work, we showed how the simultaneous resolution of two racemic compounds can be realized through the formation of chiral conglomerate cocrystals. The crystallization of a compound as a conglomerate is a rather rare phenomenon. In this study, we decided to test the probability of multi-component conglomerate formation between two racemic compounds by cocrystallizing racemic compounds with each other. Specifically, racemic proline, mandelic acid and etiracetam were cocrystallized with 14 racemic coformers. The analysis of XRPD patterns upon grinding revealed 16 potential cocrystals, four of which have been already published. The crystal structures of all new cocrystals were determined by single crystal diffraction. The crystal structure elucidation showed 13 cocrystals were racemates and 3 were conglomerates. All conglomerates are isomorphous as they were formed between etiracetam and mandelic acid or its 2-fluoro- and 2-chloro-derivatives. Simultaneous resolution through preferential cocrystallization of racemic compounds, therefore, is limited to very specific systems. In parallel, our results did, however, highlight a striking success rate of DL-proline in the context of cocrystallization as 11 out of 14 cocrystallization experiments were successful.


 Please wait while we load your content...
Please wait while we load your content...