Orientation of carbonyl groups in inclusion crystals formed from ketones with aromatic diimide-based macrocycles†
Abstract
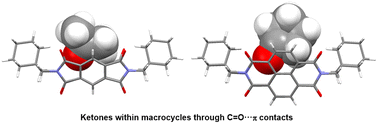
Two adamantane-bearing macrocycles (1, 2) with pyromellitic diimide or naphthalenediimide units were utilized as host molecules to elucidate the intermolecular interactions between the oxygen atoms of guest ketones and the electron-deficient π-surface of aromatic diimide moieties. Crystallization of 1 in acetone gave crystal 1a with acetone. Guest acetone molecules were captured within the cavity of 1, where C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯π contacts between the oxygen atoms of acetone and the pyromellitic diimide units were observed. From the molecular electrostatic potentials on the Hirshfeld surfaces, it was clarified that lone pair⋯π interactions predominantly contributed to the C
O⋯π contacts between the oxygen atoms of acetone and the pyromellitic diimide units were observed. From the molecular electrostatic potentials on the Hirshfeld surfaces, it was clarified that lone pair⋯π interactions predominantly contributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯π contacts. Crystallization of a dichloromethane solution of macrocycles and other ketones furnished crystals 1b with cyclopentanone, 1c with 3-oxetanone and dichloromethane, and 2a with cyclopentanone. The orientation of the carbonyl groups of ketones within the macrocycles in crystals 1b and 2a was similar to that in crystal 1a, whereas 3-oxetanone was positioned on the exterior of 1 in crystal 1c.
O⋯π contacts. Crystallization of a dichloromethane solution of macrocycles and other ketones furnished crystals 1b with cyclopentanone, 1c with 3-oxetanone and dichloromethane, and 2a with cyclopentanone. The orientation of the carbonyl groups of ketones within the macrocycles in crystals 1b and 2a was similar to that in crystal 1a, whereas 3-oxetanone was positioned on the exterior of 1 in crystal 1c.


 Please wait while we load your content...
Please wait while we load your content...