1,2-Fluorosulfenylation of unactivated alkenes with thiols and a fluoride source promoted by bromodimethylsulfonium bromide†
Abstract
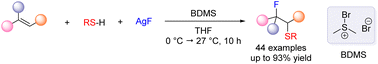
A practical method that enables the fluorosulfenylation of unactivated alkenes processed directly with thiols and fluoride salts is presented. Good to excellent efficiencies and functional group tolerance are observed for both alkene substrates and thiols. The procedure also allows the use of gaseous ethylene as a two-carbon building block for β-fluoro thioether products.


 Please wait while we load your content...
Please wait while we load your content...