CuBr-mediated synthesis of 1,4-naphthoquinones via ring expansion of 2-aryl-1,3-indandiones†
Abstract
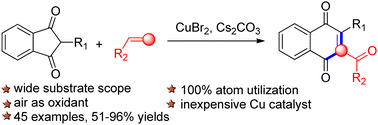
A CuBr-mediated direct insertion of alkenes into unstrained ring 2-aryl-1,3-indandiones is reported, which provides a one-carbon ring expansion strategy for the synthesis of 1,4-naphthoquinones. Entirely differing from the existing reports, the alkenes herein behave as C1 units to participate in annulation reactions. This transformation provides a facile route to access a class of highly functionalized 1,4-naphthoquinones with good yields, good functional-group tolerance and high atom-economy. Further research on the application of this reaction is currently underway in our laboratory.


 Please wait while we load your content...
Please wait while we load your content...