Phosphine-catalyzed Rauhut–Currier reaction of γ-alkyl allenoate and subsequent trapping using the Diels–Alder reaction†
Abstract
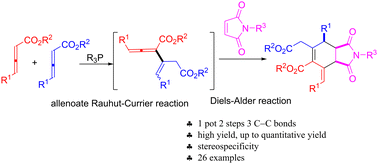
We have disclosed a Rauhut–Currier reaction of γ-alkyl-substituted allenoate, catalyzed by L-valine-derived amide phosphine, to form trisubstitued allenoate, which was trapped by maleimide or DMAD via the Diels–Alder reaction. Exo-bicyclic succinimide derivatives including three continuous stereocenters with an exo-carbon–carbon double bound were constructed in up to quantitative yields with high stereospecificity.


 Please wait while we load your content...
Please wait while we load your content...