Ruthenium-catalyzed dehydrogenative cyclization to synthesize polysubstituted 4-quinolones under solvent-free conditions†
Abstract
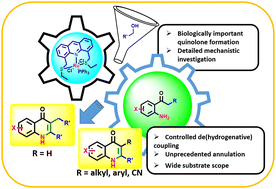
Herein, we describe acridine-based SNS-Ru pincer-catalysed unprecedented dehydrogenative annulation of alcohols with 2′-aminoacetophenone to synthesize 2,3-disubstituted-4-quinolones. The developed protocol was utilized with a wide range of alcohols with various aminoacetophenones. To expand the synthetic utility, 4-quinolones with antibiotic properties were synthesized and various important post-synthetic modifications of the synthesized scaffolds were performed. Various control experiments were performed to understand the mechanism, which showed that C-alkylation has the edge over N-alkylation and referred to the possibility of in situ alkenylation to branched ketones.


 Please wait while we load your content...
Please wait while we load your content...