Towards “homeopathic” palladium-catalysed alkoxycarbonylation of aliphatic and aromatic olefins†
Abstract
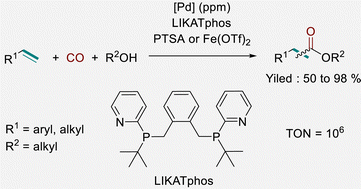
Palladium-catalysed alkoxycarbonylation of alkenes allows for atom-efficient synthesis of esters from easily available alkenes in an industrially viable manner. One of the major costs associated with this process is the consumption of the catalyst system. Hence, for economic and ecologic reasons it is desirable to minimize the amount of metal and ligands wherever possible. Herein, we report “a homeopathic” palladium-catalysed alkoxycarbonylation of olefins under comparably mild conditions. The key to success is the homemade ligand LIKATphos providing good to excellent yields of ester products with catalyst turnover numbers in the range of 106.


 Please wait while we load your content...
Please wait while we load your content...