Expedient (3+3)-annulation of in situ generated azaoxyallyl cations with diaziridines†
Abstract
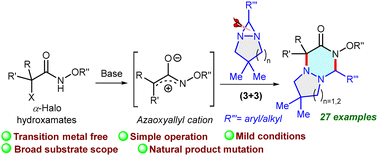
Efficient annulation of in situ formed azaoxyallyl cations using a base has been accomplished with diaziridines to provide 1,2,4-triazines at room temperature. The substrate scope, scale up, functional group tolerance and transition-metal free reaction conditions are the important practical features.


 Please wait while we load your content...
Please wait while we load your content...