PIDA-mediated N–N bond formation to access pyrazolidine-3,5-diones: a novel process for uricosuric agents G-25671 and sulfinpyrazone†
Abstract
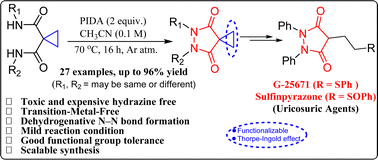
Traditionally, toxic and expensive hydrazine building blocks are required to construct pharmaceutically important pyrazolidine-3,5-diones. Herein, we have described a novel method for their synthesis based on metal-free oxidative dehydrogenative N–N bond formation by PIDA-mediated reaction of easily accessible dianilide precursors. The developed mild reaction protocol features a good functional group tolerance and scalability. The application of this method is demonstrated by offering a unique route for the synthesis of uricosuric agents G-25671 and sulfinpyrazone from inexpensive starting material aniline via smooth functionalization of the well-designed diversity-oriented cyclopropyl key intermediate.


 Please wait while we load your content...
Please wait while we load your content...