Asymmetric synthesis of highly sterically congested α-tertiary amines via organocatalyzed kinetic resolution†
Abstract
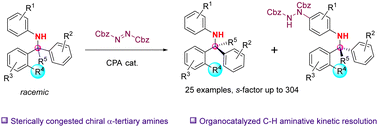
The asymmetric synthesis of highly sterically congested α-tertiary amines was achieved by an organocatalyzed kinetic resolution (KR) protocol, which were otherwise difficult to access. A variety of substituted N-aryl α-tertiary amines bearing 2-substitued phenyl groups were kinetically resolved through the asymmetric C–H amination reaction, affording good to high KR performances.
- This article is part of the themed collection: 2023 Pioneering Investigators


 Please wait while we load your content...
Please wait while we load your content...