Sustainable preparation of aminosilane monomers, oligomers, and polymers through Si–N dehydrocoupling catalysis
Abstract
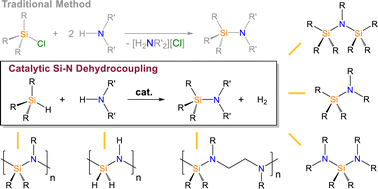
This article covers historical and recent efforts to catalyse the dehydrocoupling of amines and silanes, a direct method for Si–N bond formation that offers hydrogen as a byproduct. In some applications, this transformation can be used as a sustainable replacement for traditional aminosilane synthesis, which demands corrosive chlorosilanes while generating one equivalent of ammonium salt waste for each Si–N bond that is formed. These advantages have driven the development of Si–N dehydrocoupling catalysts that span the periodic table, affording mechanistic insight that has led to advances in efficiency and selectivity. Given the divergence in precursors being used, characterization methods being relied on, and applications being targeted, this article highlights the formation of monomeric aminosilanes separately from oligomeric and polymeric aminosilanes. A recent study that allowed for the manganese catalysed synthesis of perhydropolysilazane and commercial chemical vapor deposition precursors is featured, and key opportunities for advancing the field of Si–N dehydrocoupling catalysis are discussed.


 Please wait while we load your content...
Please wait while we load your content...