Electrochemical synthesis of 2-alkyl-4-phenylalkan-2-ols via cathodic reductive coupling of alkynes with unactivated aliphatic ketones†
Abstract
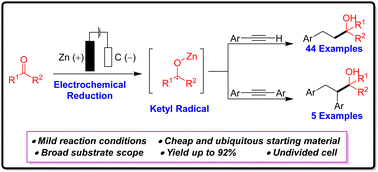
Herein, we present an efficient electrochemical method for synthesizing 2-alkyl-4-phenylalkan-2-ols through an electrochemically driven cathodic reductive coupling of the terminal and internal acetylenes with unactivated aliphatic ketones under mild conditions. The process proceeds through a ketyl radical, which then activates the aryl acetylene and causes complete reduction of the triple bond of the acetylene moiety. This strategy is environmentally benign and exhibits a broad substrate scope with ubiquitously available starting materials.


 Please wait while we load your content...
Please wait while we load your content...