Non-covalent interactions reveal the protein chain δ conformation in a flexible single-residue model†
Abstract
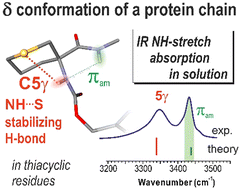
The δ conformation is a local secondary structure in proteins that implicates a πamide N–H⋯N interaction between a backbone N atom and the NH of the following residue. Small-molecule models thereof have been limited so far to rigid proline-type compounds. We show here that in derivatives of a cyclic amino acid with a sulphur atom in the γ-position, specific side-chain/backbone N–H⋯S interactions stabilize the δ conformation sufficiently to allow it to compete with classical C5 and C7 H-bonded conformers.


 Please wait while we load your content...
Please wait while we load your content...