Photoreductive β-aminoalkylation with amino acids affords functionalized γ-aminoketones for nucleoside mimics†
Abstract
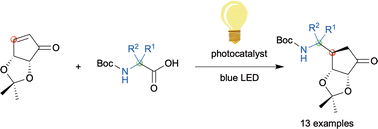
We developed a facile photoreductive and stereoselective β-aminoalkylation of a crowded enone by blue LED light irradiation using a wide variety of α-amino acids in order to access 5′-amino substituted carbasugar nucleosides for SAM-based methyltransferase inhibitors. This photochemical method provides highly functionalized carbasugar mimics for nucleoside analogue synthesis.


 Please wait while we load your content...
Please wait while we load your content...