Organo-cyanamides: convenient reagents for catalytic amidation of carboxylic acids†
Abstract
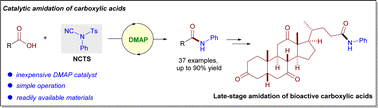
An unprecedented DMAP-catalysed amidation of aryl and alkyl carboxylic acids with organo-cyanamides has been developed. Unlike the use of N-cyano-N-phenyl-p-methylbenzenesulfonamide (NCTS) as an electrophilic cyanating reagent, an unusual desulfonylation/decyanation reaction model has been disclosed for the first time. Remarkable features of this reaction include readily available substrates, simple operation and broad scope, enabling the efficient synthesis of structurally diverse amides. The synthetic utility of this protocol was demonstrated by the late-stage amidation of bioactive carboxylic acids and a scale-up reaction.


 Please wait while we load your content...
Please wait while we load your content...