Biological formation of ethylene
Abstract
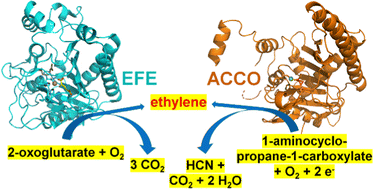
This review summarizes the structures, biochemical properties, and mechanisms of two major biological sources of ethylene, the ethylene-forming enzyme (EFE) and 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase (ACCO). EFE is found in selected bacteria and fungi where it catalyzes two reactions: (1) the oxygen-dependent conversion of 2-oxoglutarate (2OG) to ethylene plus three molecules of CO2/bicarbonate and (2) the oxidative decarboxylation of 2OG while transforming L-arginine to guanidine and L-Δ1-pyrroline-5-carboxylic acid. ACCO is present in plants where it makes the plant hormone by transforming ACC, O2, and an external reductant to ethylene, HCN, CO2, and water. Despite catalyzing distinct chemical reactions, EFE and ACCO are related in sequence and structure, and both enzymes require Fe(II) for their activity. Advances in our understanding of EFE, derived from both experimental and computational approaches, have clarified how this enzyme catalyzes its dual reactions. Drawing on the published mechanistic studies of ACCO and noting the parallels between this enzyme and EFE, we propose a novel reaction mechanism for ACCO.


 Please wait while we load your content...
Please wait while we load your content...