Emulating nonribosomal peptides with ribosomal biosynthetic strategies
Abstract
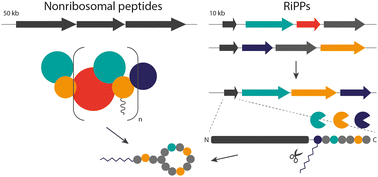
Peptide natural products are important lead structures for human drugs and many nonribosomal peptides possess antibiotic activity. This makes them interesting targets for engineering approaches to generate peptide analogues with, for example, increased bioactivities. Nonribosomal peptides are produced by huge mega-enzyme complexes in an assembly-line like manner, and hence, these biosynthetic pathways are challenging to engineer. In the past decade, more and more structural features thought to be unique to nonribosomal peptides were found in ribosomally synthesised and posttranslationally modified peptides as well. These streamlined ribosomal pathways with modifying enzymes that are often promiscuous and with gene-encoded precursor proteins that can be modified easily, offer several advantages to produce designer peptides. This review aims to provide an overview of recent progress in this emerging research area by comparing structural features common to both nonribosomal and ribosomally synthesised and posttranslationally modified peptides in the first part and highlighting synthetic biology strategies for emulating nonribosomal peptides by ribosomal pathway engineering in the second part.


 Please wait while we load your content...
Please wait while we load your content...