Design of an apoptotic cell-mimetic wound dressing using phosphoserine–chitosan hydrogels†
Abstract
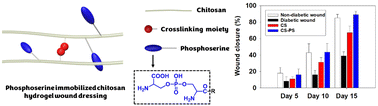
Inflammatory M1 macrophages create a hostile environment that impedes wound healing. Phosphoserine (PS) is a naturally occurring immunosuppressive molecule capable of polarizing macrophages from an inflammatory phenotype (M1) to an anti-inflammatory phenotype (M2). In this study, we designed, fabricated, and characterized PS-immobilized chitosan hydrogels as potential wound dressing materials. A PS group precursor was synthesized via a phosphoramidite reaction and subsequently immobilized onto the chitosan chain through an EDC/N-hydroxysuccinimide reaction using a crosslink moiety HPA. The PS/HPA-conjugated chitosan (CS–PS) was successfully synthesized by deprotecting the PS group in HCl. In addition, the hydrogels were prepared by the HRP/H2O2 enzyme-catalyzed reaction with different PS group contents (0, 7.27, 44.28 and 56.88 μmol g−1). The immobilization of the PS group improved the hydrophilicity of the hydrogels. Interestingly, CS–PS hydrogel treatment upregulated both pro-inflammatory and anti-inflammatory cytokines. This treatment also resulted in alterations in the macrophage cell morphology from the M1 to M2 phenotype. The CS–PS hydrogel significantly accelerated diabetic wound healing. Overall, this study provides insights into the potential of PS-immobilized hydrogel materials for improved inflammatory disease therapy.


 Please wait while we load your content...
Please wait while we load your content...