Determination of vitamin D3 conjugated metabolites: a complementary view on hydroxylated metabolites†
Abstract
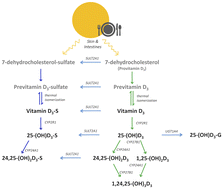
Experts typically define vitamin D deficiency levels by the determination of a circulating 25-hydroxyvitamin D3—calcifediol prohormone. A large part of the population is characterized by deficient vitamin D levels (calcifediol < 20 ng mL−1) despite individuals not being affected by any disorder. Cholecalciferol (vitamin D3) and/or calcifediol supplementation is a common practice for vitamin D-deficient individuals as recommended by international scientific societies and official agencies. In the last few years, several studies have reported the presence of conjugated vitamin D3 metabolites, mainly glucuronidation and sulfation derivatives, although simultaneous quantitative measurements involving phase I and II vitamin D metabolites have not been carried out. A quantitative method based on tandem mass spectrometry detection is proposed here for the combined determination of phase I and phase II vitamin D3 metabolites in human serum. As phase I and phase II metabolites are preferentially ionized in different modes, a switching polarity mode was adopted to determine both groups of compounds in serum at high sensitivity levels (pg mL−1). The validation of this proposal was successfully accomplished by following the Center for Drug Evaluation and Research (CDER) guidelines. Its applicability was tested in a cohort of volunteers with mostly deficient baseline levels. Considering the sulfated form of calcifediol, the sum of its concentrations showed sufficient baseline vitamin D levels in all individuals, suggesting that this could be a novel strategy for vitamin D deficiency definition. Therefore, phase II metabolites are proposed to be included when evaluating the vitamin D status since they provide more information about the overall status of the vitamin D endocrine system. Nevertheless, further studies are required to confirm the biological activity of these conjugated metabolites and the suitability of this strategy for the description of vitamin D deficiency.


 Please wait while we load your content...
Please wait while we load your content...