A mild synthetic strategy for removing acetic acid from fast pyrolysis-derived bio-oils utilizing Friedel–Crafts acylation reactions†
Abstract
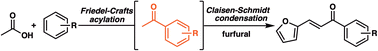
Fast pyrolysis-derived bio-oils contain numerous oxygenated components, including acetic acid and formic acid. However, these acids present in fast pyrolysis-derived bio-oils are responsible for the instability and corrosiveness of the oils. Although these acids have been removed from fast pyrolysis-derived bio-oils by esterification with alcohols to form acetates, these acetates could not be transformed to compounds with a suitable number of carbon atoms required to be used as fuels. In this work, Friedel–Crafts acylation reactions were conducted to remove acetic acid to form acetophenones, which possess more than eight carbons and could be converted to valuable components of fuels.
- This article is part of the themed collection: Energy Advances: Highlight Japan & South Korea


 Please wait while we load your content...
Please wait while we load your content...