Thermal decomposition of ammonium nitrate (AN) in the presence of the optimized nano-ternary transition metal ferrite CoNiZnFe2O4†
Abstract
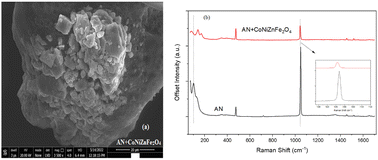
CoNiZnFe2O4 was synthesized and characterized using techniques such as powder XRD, Raman spectroscopy, and infrared (IR) spectroscopy. The optical band-gap energy and thermal stability of CoNiZnFe2O4 were also explored. The effect of 2% by mass of CoNiZnFe2O4 on the thermal decomposition of ammonium nitrate (AN) was studied using simultaneous thermal analysis (TG-DTA). The presence of CoNiZnFe2O4 in AN converts the endothermic curve of AN into an exothermic curve with a reduction in the peak temperature by 20 °C. The kinetic investigations suggest that the thermal decomposition of AN + CoNiZnFe2O4 occurs at a lower activation energy (by 76 kJ mol−1) with a decreased pre-exponential factor (by 0.4 min−1), suggesting a faster decomposition of AN in the presence of CoNiZnFe2O4. The findings of the present work suggest that AN + CoNiZnFe2O4 can be used in place of pure AN as an oxidizer in military applications.


 Please wait while we load your content...
Please wait while we load your content...