Effect of doping TiO2 with Mn for electrocatalytic oxidation in acid and alkaline electrolytes†
Abstract
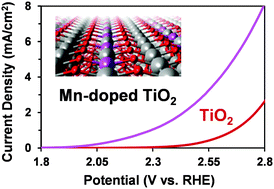
Electrochemical oxidation of water and electrolyte ions is a sustainable method for producing energy carriers and valuable chemicals. Among known materials for catalyzing oxidation reactions, titanium dioxide (TiO2) offers excellent electrochemical stability but is less active than many other metal oxides. Herein, we used density functional theory calculations to predict an increase in catalytic activity by doping anatase TiO2 with manganese atoms (Mn). We synthesized Mn-doped TiO2 and then utilized X-ray absorption spectroscopy to study the chemical environment around the Mn site in the TiO2 crystal structure. Our electrochemical experiments confirmed that TiO2, with the optimal amount of Mn, reduces the onset potential by 260 mV in a 2 M KHCO3 (pH = ∼8) electrolyte and 370 mV in a 0.5 M H2SO4 (pH = ∼0.5) electrolyte. Moreover, in 0.5 M H2SO4, we observed that the amount of Mn doping greatly impacts the selectivity towards oxygen production versus peroxysulfate formation. In 2 M KHCO3, the Mn doping of TiO2 slightly decreases the selectivity towards oxygen production and increases the hydrogen peroxide formation. The Mn-doped TiO2 shows good electrochemical stability for over 24 hours in both electrolytes.


 Please wait while we load your content...
Please wait while we load your content...