A localized high concentration electrolyte for 4 V-class potassium metal batteries†
Abstract
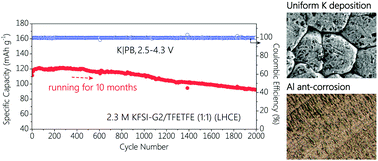
Rechargeable potassium-metal batteries (PMBs) meet the need for high energy density because the potassium (K) metal anode has the highest specific capacity (687 mA h g−1) among anode materials, but electrolytes that are stable with both the highly reactive K metal anode and high-voltage cathodes remain a serious challenge. Here, we report a localized high concentration electrolyte consisting of potassium bis(fluoroslufonyl)imide (KFSI) in diethylene glycol dimethyl ether (G2) with 1,1,2,2-tetrafluoro-1-(2,2,2-trifluoroethoxy)ethane (TFETFE) (1 : 1.5 : 1.5) as a diluent for 4 V-class PMBs. This new electrolyte shows unprecedented cycling stability in K|Prussian blue (PB) batteries with a capacity retention of 83% over 2000 cycles (over 10 months) at a charge cut-off voltage of 4.3 V. Higher voltage tolerance of 4.4 V is also confirmed in the PMBs for 300 cycles. This study provides an avenue to design electrolytes for high-energy PMBs in the future.
- This article is part of the themed collections: Energy Advances: Highlight China and Energy Advances 2022 Hot Papers


 Please wait while we load your content...
Please wait while we load your content...