Effect of Hofmeister ions on the spectro- and electrochemical performance of PANI†
Abstract
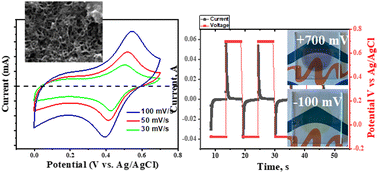
Investigation of energy accumulation systems has remained a promising strategy for many decades. Li-containing devices have the first place in such systems and have several advantages. However, these systems could be replaced by Al-containing ones, which are relatively new due to the abundance of the element aluminum, low cost, and its high capacity. Herein, we present a new, general, and robust approach to using Al-ions for the accumulation of energy in an organic polymer–polyaniline (PANI). The synergistic effect of the stability of the PANI polymer and Al-containing electrolytes is studied. First, PANI was electrochemically prepared in the presence of formic acid, which can form hydrogen bonding with polymer chains that leads to high organization of the chains; and its morphology and chemical structure were characterized by scanning electron microscopy, X-ray photoelectron spectroscopy, and Raman spectroscopy. The electrochemical performance of PANI/Al(ClO4)3 was studied by cyclic voltammetry and electrochemical impedance spectroscopy. The PANI film revealed a reversible one reduction and one oxidation peak, which is explained by the organization of the polymer chains on one hand, and the application of Al(ClO4)3 as the electrolyte on the other hand. We have found that the PANI/Al(ClO4)3 system exhibits a significant improvement in electrochemical activity and stability (after 5000 cycles ∼90% of the original performance is retained), and improved spectro-electrochemical performance: the optical contrast ratio is 6.25 and coloration efficiency is 102.7 cm2 C−1 at 633 nm.


 Please wait while we load your content...
Please wait while we load your content...