Activity and selectivity of N2 fixation on B doped g-C9N10: a density functional theory study†
Abstract
N2 fixation driven by photocatalysis or electrocatalysis to produce ammonia under mild conditions is a promising method to replace the industrial Haber–Bosch process. Inspired by recent studies, which showed that the boron atom can effectively activate N2 due to its Lewis-acid characteristics, we herein investigate the mechanism of N2 adsorption and fixation on B doped g-C9N10, a new carbon nitride material, with three different doping configurations, namely substitutions of B at C (BC1) and N (BN1) sites and B anchored g-C9N10 (BA) by density functional theory calculations. We found that the nitrogen reduction reaction (N2RR) can only proceed on BN1 and BA due to N2 chemisorption ability. The optimal N2RR mechanism of the BN1 and BA doped g-C9N10 is the mix I mechanism 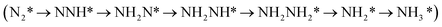 with mix I low limiting potential of −0.62 V and −0.44 V, respectively. However, the H poisoning effect at BA doped g-C9N10 due to stronger H adsorption than N2 adsorption will suppress N2RR selectivity. In contrast, H poisoning at BN1 doped g-C9N10 can be effectively inhibited due to weaker H adsorption ability, thereby improving the selectivity for the N2RR. The electronic structure analysis indicates that the H–B interaction arises from hybridization between B 2py and H 1s orbitals, which is suppressed in the case of BN1 because the 2py orbital of is less populated BN1. Our work provides useful guidance for more experimental works to explore more B doped carbon nitride materials for the N2 fixation field.
with mix I low limiting potential of −0.62 V and −0.44 V, respectively. However, the H poisoning effect at BA doped g-C9N10 due to stronger H adsorption than N2 adsorption will suppress N2RR selectivity. In contrast, H poisoning at BN1 doped g-C9N10 can be effectively inhibited due to weaker H adsorption ability, thereby improving the selectivity for the N2RR. The electronic structure analysis indicates that the H–B interaction arises from hybridization between B 2py and H 1s orbitals, which is suppressed in the case of BN1 because the 2py orbital of is less populated BN1. Our work provides useful guidance for more experimental works to explore more B doped carbon nitride materials for the N2 fixation field.



 Please wait while we load your content...
Please wait while we load your content...