Modulating the photoelectrocatalytic conversion of CO2 to methanol and/or H2O to hydrogen at a phosphorene modified Ti/TiO2 electrode†
Abstract
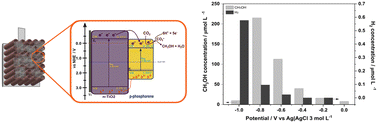
The effect of phosphorene as a co-catalyst for Ti/TiO2 nanotube electrodes on CO2 reduction to methanol, and water splitting was investigated. The electrode was prepared by using poly-dopamine as an anchoring agent to attach phosphorene, which was initially prepared by liquid exfoliation from black phosphorous. In this photoelectrocatalytic system, phosphorene acts as a p-type semiconductor with an energy band gap of −1.46 eV and strong photoactivation under visible light irradiation. Under optimized conditions, solar simulator irradiation and applied potential of −0.8 V in 0.1 mol L−1 Na2SO4 and pH 7 the CO2 reduction is preponderant in the process. The electrode system was found to also promote the production of hydrogen from water reduction when the photoelectrocatalytic process is conducted at the applied potential of −1.0 V in 0.1 mol L−1 Na2SO4 (pH 2). The results indicate that phosphorene can be a good modifier of Ti/TiO2, and the high charge mobility amplify its potential applicability to CO2 conversion.
- This article is part of the themed collection: In Memoriam of Prof. Richard T. Williams (May 27, 1946 - July 5, 2021)


 Please wait while we load your content...
Please wait while we load your content...