Substituent effects on the intermolecular interactions and emission behaviors in pyrene-based mechanochromic luminogens†
Abstract
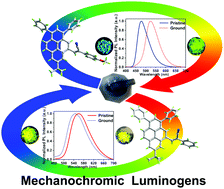
Mechanochromic luminogenic (MCL) materials have always been scientifically intriguing owing to their promising application prospects in mechanosensor, optical storage and optoelectronic devices. In the present work, two cyanoethylene-functionalized pyrene-based luminogens 3a and 3b were designed and synthesized. The experimental results show that 3a and 3b exhibit distinct photophysical properties in dilute solution and in various solid states. The substituent effect is even more obvious with regard to their mechanochromic properties. A blue-shift for 3a (12 nm) and a remarkable red-shift for 3b (47 nm) were observed with mechanical stimuli. The intriguing emission behaviour was further explored by in-depth theoretical simulations combined with molecular packing morphology based on X-ray crystallography. These results indicate that the significant inter-layer slippage upon grinding in 3a contributes to the blue-shift and enhanced emission by weakening the π⋯π interactions, while the collapsed intermolecular interactions in 3b through mechanical stimulation give rise to the red-shifted and attenuated luminescence by strengthening the molecular motion freedom and planar configuration. The present work provides a valuable strategy to construct pyrene-based MCL materials based on the substituent effect.


 Please wait while we load your content...
Please wait while we load your content...