Helical thienothiophene (TT) and benzothieno–benzothiophene (BTBT) derivatives: synthesis, structural characterization and semiconducting properties†
Abstract
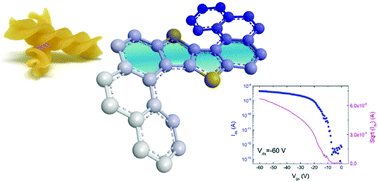
Thienothiophene (TT) and benzothieno–benzothiophene (BTBT) have been successfully included here in the helical backbone of helicene derivatives. The employed synthetic approach gives access in a controlled manner to both simple and double helicenic structures decorated with side alkyl chains to provide solubility. Whereas in the solid state structure the M and P forms of the mono-helicene stack in alternating rows of each enantiomer and show segregation between the aromatic and aliphatic parts, the crystal of the bis-helicene is formed of homochiral sheets of MM and PP enantiomers. These helical TT and BTBT materials have been tested as racemic mixtures in organic field-effect transistor (OFET) devices fabricated by both spin coating and vapour deposition. The bis-helicene derivative, in which crystal packing is dominated by π–π stacking interactions, behaves as a p-type semiconductor with a hole mobility of 3.5 × 10−5 cm2 V−1 s−1 in close agreement with the predicted value by DFT calculations. The OFETs of mono-helicene do not show charge transport despite the superior predicted mobility based on the crystal structure, suggesting that amorphous films suffer from a broader distribution of hole energies, which limits the number of thermally accessible hopping pathways. The benefits of embedding TT and BTBT units into helicenic structures in terms of synthetic strategy, structural variation and mobility pave the way for chiral semiconducting BTBT helicenes.


 Please wait while we load your content...
Please wait while we load your content...