One F atom matters: synthesis, aggregation-induced emission and stimuli-responsiveness of three isomers of fluoro/formyl substituted tetraphenylethene derivatives†
Abstract
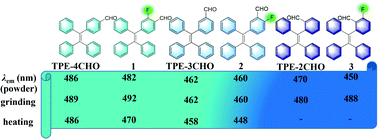
By introducing fluorine atom(s) into luminescent organic compounds, it may be possible to fine-tune their packing structure and luminescence properties. In the present work, three isomers of fluoro/formyl substituted tetraphenylethene (TPE) derivatives (1–3) with one fluorine atom have been synthesized via the Suzuki–Miyaura cross-coupling reactions in high yields. With the different substitution positions of fluoro/formyl substituents, compounds 1–3 exhibit different luminescence behavior in THF/H2O mixed solvents and solid state, as well as responsiveness towards mechanical force and heat when compared with the non-fluorinated analogs TPE-4CHO, TPE-3CHO and TPE-2CHO. Compounds 1 and 3 exhibit mechanofluorochromism with red-shifted emissions, more drastic than those of TPE-4CHO and TPE-2CHO, while 2 and TPE-3CHO do not show this property under the same conditions. Compounds 1–3 all show thermofluorochromism with weaker and blue-shifted emissions on increasing temperatures, different from those of the non-fluorinated analogs, which only exhibit weaker emissions without a wavelength shift under the same conditions. Furthermore, the three compounds form composites with silica gel or filter paper strips, and the resulting composites show stronger and red-shifted emissions compared to the corresponding compounds, and also exhibit thermofluorochromism, which has potential in fluorescent thermometers and anti-counterfeiting.


 Please wait while we load your content...
Please wait while we load your content...