Circularly polarised luminescence in an RNA-based homochiral, self-repairing, coordination polymer hydrogel†
Abstract
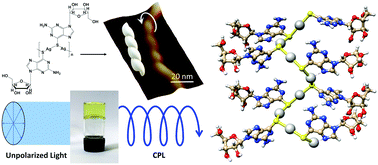
The aqueous equimolar reaction of Ag(I) ions with the thionucleoside enantiomer (−)6-thioguanosine, ((−)6tGH), yields a one-dimensional coordination polymer {Ag(−)tG}n, the self-assembly of which generates left-handed helical chains. The resulting helicity induces an enhanced chiro-optical response compared to the parent ligand. DFT calculations indicate that this enhancement is due to delocalisation of the excited state along the helical chains, with 7 units being required to converge the calculated CD spectra. At concentrations ≥15 mmol l−1 reactions form a sample-spanning hydrogel which shows self-repair capabilities with instantaneous recovery in which the dynamic reversibility of the coordination chains appears to play a role. The resulting gel exhibits circularly polarised luminescence (CPL) with a large dissymmetry factor of −0.07 ± 0.01 at 735 nm, a phenomenon not previously observed for this class of coordination polymer.
- This article is part of the themed collection: Celebrating our 2025 Prizewinners


 Please wait while we load your content...
Please wait while we load your content...