Competing ferroelectric polarization: hydroxyl flip-flop versus proton-transfer mechanisms†
Abstract
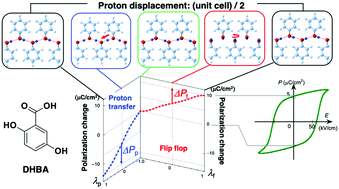
Ferroelectricity above room temperature and a theoretical simulation of the spontaneous polarization are reported for a polar polymorph of 2,5-dihydroxybenzoic acid. The planar zigzag hydrogen-bonded chain of hydroxy groups geometrically permits two distinct mechanisms: flip-flop motion of hydroxy groups and concerted intermolecular proton transfer. Density functional theory calculations indicate that the polarizations of these two mechanisms are quite different in magnitude with opposite sign. Both the experimental and theoretical investigations are consistently in favor of a flip-flop process: it is more favorable than the proton transfer in energy and quantitatively explains the observed spontaneous polarization. Flip-flop type polarization reversal is also supported by the minimal effects of deuteration on the ferroelectric properties and stability.


 Please wait while we load your content...
Please wait while we load your content...