Enhancing ionic conductivity in tablet–bottlebrush block copolymer electrolytes with well-aligned nanostructures via solvent vapor annealing†
Abstract
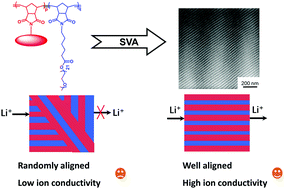
Block copolymer electrolytes (BCPEs) are one of the most attractive alternatives to traditional liquid electrolytes in lithium-ion batteries. A series of relatively narrowly distributed tablet–bottlebrush (TB) block copolymer electrolytes, which have mesogen-jacketed liquid crystalline polymer segments and polyethylene oxide (PEO) side chains, were synthesized through tandem ring-opening metathesis polymerization. After doping with lithium salts, these BCPEs self-assembled into different microphase-separated structures. The lamellar structure could be oriented to be perpendicular to the substrate after solvent vapor annealing (SVA) in a long range of more than 4 μm. The well-aligned films are as thick as several hundred microns, which is different from previous reports on thin-film alignment. The effect of orientation on the ion conductivity was investigated. Enhanced by the orientation, the ion conductivity of the well-aligned BCPE membranes was 2.19 mS cm−1 at 200 °C. The high stability and impressive ion conductivity at high temperatures make the TB-based BCPEs a promising electrolyte candidate for high-temperature lithium ion batteries.


 Please wait while we load your content...
Please wait while we load your content...