Molecular design to enhance binaphthyl-based chiroptics using organoboron chemistry in isomeric chiral scaffolds†
Abstract
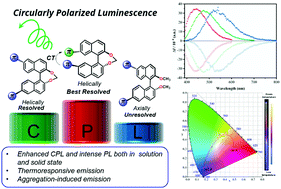
Access to high-performance circularly polarized luminescence is highly desired in materials science but it hitherto remains a considerable challenge. We herein present a conceptually universal design strategy to improve the chiral luminescent properties of a series of binaphthyl-based compounds (MeBTT, MeBTB, p-BTT, p-BTB, m-BTT and m-BTB). The binaphthyl skeleton in all cases was π-functionalized either by an electron donor (Ar3N) or acceptor (Ar3B), and correlations of the molecular structures to their photophysical characters were systematically investigated. They all exhibited strong photoluminescence both in solution with quantum efficiency (ΦPL,DCM) up to 100% and as solids (ΦPL,solid = 21–59%). The optical resolution into enantiomers via chiral HPLC was achieved for p-BTT, p-BTB, m-BTT and m-BTB with helically ring-structured binaphthyls. We further unveiled that the sterically more constrained m-BTT and m-BTB exhibit superior chiroptical properties in circularly polarized luminescence (CPL) relative to the isomers p-BTT and p-BTB, evident from the order of magnitude of luminescence dissymmetry factor (|glum|) increasing from 10−4 to 10−3 in solution. Moreover, the double functionalization of the binaphthyl moiety with an electron donor–acceptor charge-transfer system resulted also in an experimentally improved CPL activity, as rationalized by TD-DFT calculations of a key angle (θμ,m) between vectors of the electric and magnetic transition dipole moments in the excited states. The three organoboranes (MeBTB, p-BTB and m-BTB) displayed dual emissions in polar solvents and the low-energy charge transfer bands were demonstrated to be air-sensitive and thermally responsive at elevated temperature.


 Please wait while we load your content...
Please wait while we load your content...