Enhanced blue TADF in a D–A–D type naphthyridine derivative with an asymmetric carbazole-donor motif†
Abstract
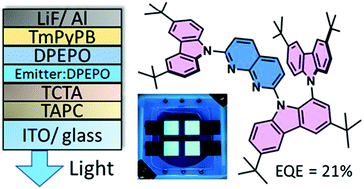
Efficient triplet-to-singlet conversion in conventional donor–acceptor TADF compounds relies on charge-transfer (CT) and locally-excited (LE) triplet state mixing as this provides the required spin–orbit coupling. In this work, an asymmetric carbazole-donor motif is shown to facilitate the coupling and spin–flip process in a naphthyridine-acceptor based TADF emitter due to the enhanced LE character of the lowest triplet state. This was revealed from the delayed fluorescence measurements, which clearly signified a doubled RISC rate (up to 1.33 × 106 s−1) and a shortened delayed fluorescence lifetime (down to 4.4 μs) in an asymmetric compound compared to those of symmetric or singly-carbazole-substituted compounds. The promising features of the asymmetric blue TADF emitter were demonstrated to result in high efficiency OLEDs (external quantum efficiency of 21%) with a reduced efficiency roll-off.
- This article is part of the themed collection: Materials for thermally activated delayed fluorescence and/or triplet fusion upconversion


 Please wait while we load your content...
Please wait while we load your content...