Ferroelectricity driven by orbital resonance of protons in CH3NH3Cl and CH3NH3Br
Abstract
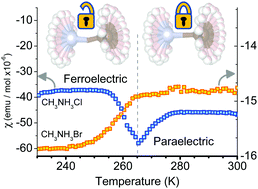
The β and γ phases of methylammonium chloride CH3NH3Cl and methylammonium bromide CH3NH3Br are identified to be ferroelectric via pyroelectric current and dielectric constant measurements. The magnetic susceptibility also exhibits pronounced discontinuities at the Curie temperatures. We attribute the origin of spontaneous polarization to the emergence of two groups of proton orbital magnetic moments from the uncorrelated motion of the CH3 and NH3 groups in the β and γ phases. The two inequivalent frameworks of intermolecular orbital resonances interact with each other to distort the lattice in a non-centrosymmetric fashion. Our findings indicate that the structural instabilities in molecular frameworks are magnetic in origin as well as providing a new pathway toward uncovering new organic ferroelectrics.


 Please wait while we load your content...
Please wait while we load your content...