The effect of molecular aggregation of thermally activated delayed fluorescence sensitizers for hyperfluorescence in organic light-emitting diodes†
Abstract
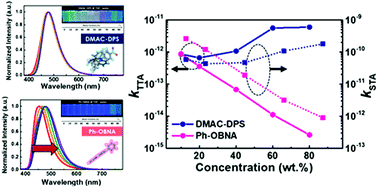
The exciton behavior of a new synthetic material, 11-phenyl-11H-9,16-dioxa-11-aza-4b-boraindeno[1,2-a]naphtho[3,2,1-de]anthracene (Ph-OBNA), which displays a multi-resonance effect, and bis[4-(9,9-dimethyl-9,10-dihydroacridine)phenyl]sulfone (DMAC-DPS) with a donor–acceptor-donor structure was analyzed. First, we conducted quantum mechanical simulations based on long-range theory (LC-ωPBE) to predict kRISC, and we confirmed the effect of the luminescence behavior by analyzing aggregation-caused quenching (ACQ) according to π–π stacking and aggregation-induced emission (AIE) behavior. Actually, thermally activated delayed fluorescence materials showing ACQ and AIE behavior exhibited different triplet–triplet annihilation (TTA) and singlet–triplet annihilation (STA) rate constants. Interestingly, DMAC-DPS exhibited a restricted exciton concentration quenching behavior based on an observed AIE owing to its inhibited molecular stacking behavior. On the other hand, Ph-OBNA demonstrated somewhat reduced TTA and STA behavior due to an ACQ behavior originating from excimer formation at high concentrations. Despite this difference, both materials demonstrated a hyperfluorescence behavior that can be used to achieve moderately high device efficiency compared with that obtained from common fluorescence emitters.
- This article is part of the themed collection: Materials for thermally activated delayed fluorescence and/or triplet fusion upconversion


 Please wait while we load your content...
Please wait while we load your content...