Conformational-specific self-assembled peptides as dual-mode, multi-target inhibitors and detectors for different amyloid proteins†
Abstract
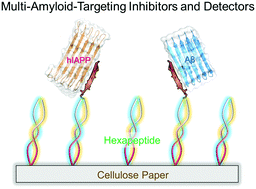
Prevention and detection of misfolded amyloid proteins and their β-structure-rich aggregates are the two promising but different (pre)clinical strategies to treat and diagnose neurodegenerative diseases including Alzheimer's diseases (AD) and type II diabetes (T2D). Conventional strategies prevent the design of new pharmaceutical molecules with both amyloid inhibition and detection functions. Here, we propose a “like-interacts-like” design principle to de novo design a series of new self-assembling peptides (SAPs), enabling them to specifically and strongly interact with conformationally similar β-sheet motifs of Aβ (association with AD) and hIAPP (association with T2D). Collective in vitro experimental data from thioflavin (ThT), atomic force microscopy (AFM), circular dichroism (CD), and cell assay demonstrate that SAPs possess two integrated functions of (i) amyloid inhibition for preventing both Aβ and hIAPP aggregation by 34–61% and reducing their induced cytotoxicity by 7.6–35.4% and (ii) amyloid sensing for early detection of toxic Aβ and hIAPP aggregates using in-house SAP-based paper sensors and SPR sensors. The presence of both amyloid inhibition and detection in SAPs stems from strong molecular interactions between amyloid aggregates and SAPs, thus providing a new multi-target model for expanding the new therapeutic potentials of SAPs and other designs with built-in amyloid inhibition and detection functions.


 Please wait while we load your content...
Please wait while we load your content...